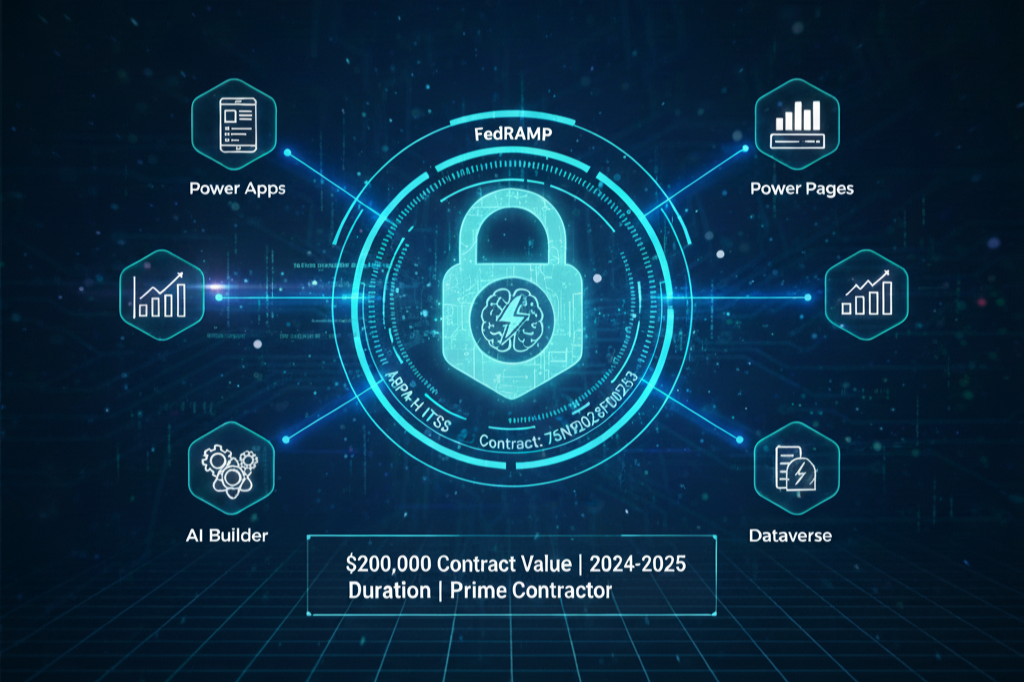
GRACE AI - SECURE GENERATIVE AI ASSISTANT
Private FedRAMP-Compliant Research Acceleration Platform
-
Advanced Research Projects Agency for Health (ARPA-H)
-
ITSS (Information Technology Support Services)
-
75N92023F00253
-
$200,000
-
2024-2025
-
Time & Materials (T&M) and Firm Fixed Price (FFP)
-
Prime Contractor (delivered through Phase No. 5)
Challenge
ARPA-H researchers and program managers needed access to advanced generative AI capabilities to accelerate biomedical research, analyze scientific literature, and synthesize complex health data. However, using commercial AI platforms like ChatGPT posed significant security risks including potential data exposure, lack of federal compliance, inability to access classified or sensitive research data, and no integration with agency-specific knowledge repositories. The agency required a secure, compliant AI solution that could enhance productivity while maintaining the highest standards of data protection and federal information security.
Solution
DAWNE IT Solutions (through Phase No. 5) designed and deployed GRACE (General Research And Content Engine), a secure, private generative AI assistant tailored specifically for ARPA-H's research mission. Named in homage to pioneering computer scientist Grace Hopper, the platform delivered ChatGPT-4 level capabilities within a FedRAMP-compliant environment with enhanced access to biomedical research databases and ARPA-H internal knowledge.
Core Capabilities
-
Dedicated, isolated AI instance for ARPA-H exclusive use
GPT-4 level natural language processing and generation
Conversational interface familiar to commercial AI users
Multi-turn dialogue with context retention
Code generation and technical documentation support
Research synthesis and summarization capabilities
Real-time inventory visibility across all ARPA-H locations
Automated tracking of equipment assignments and transfers
Government property identification decal management
Peripheral device tracking (monitors, keyboards, docking stations, etc.)
Office supply inventory monitoring and replenishment
Equipment pooling for temporary assignments
-
PubMed Integration: Direct access to 35+ million biomedical research citations
Automated literature search and retrieval
Citation extraction and bibliography generation
Research trend analysis across publications
Author and institution network mapping
HHS Data Access: Integration with Department of Health and Human Services datasets
Public health statistics and epidemiological data
Clinical trial registries and outcomes
Healthcare policy documents and regulations
Federal health program data
ARPA-H Internal Documents: Secure access to agency knowledge repositories
Internal research reports and findings
Program documentation and proposals
Meeting notes and decision records
Strategic planning documents
Policy and procedure manuals
-
Rapid literature review and synthesis
Research hypothesis generation
Experimental design recommendations
Data analysis methodology suggestions
Grant proposal drafting assistance
Scientific writing and editing support
Cross-disciplinary connection identification
Research gap analysis
-
Private cloud deployment with data isolation
FedRAMP-compliant infrastructure
End-to-end encryption for all queries and responses
No data sharing with external AI providers
Comprehensive audit logging of all interactions
Role-based access controls
Multi-factor authentication
Data residency within federal boundaries
-
Document upload and analysis
Batch processing for multiple documents
Export capabilities for research outputs
Collaboration features for team projects
Version control for generated content
Integration with Microsoft 365 environment
Technologies Used
AI Platform
GPT-4 architecture (private deployment)
Security
Multi-factor authentication, encryption at rest and in transit
Collaboration
Microsoft Teams integration for shared research
Cloud Infrastructure
FedRAMP-compliant cloud environment
Access Management
Okta SSO integration
Data Integration
PubMed API, HHS data portals, SharePoint
Monitoring
Comprehensive logging and audit trail systems
Implementation Phases
Phase 1: Architecture & Security Design
FedRAMP compliance assessment and planning
Security architecture design and documentation
Data classification and handling procedures
Access control policy development
Integration architecture planning
Phase 3: Testing & Validation
Security penetration testing
Functionality validation across use cases
Performance optimization
User acceptance testing with research teams
Compliance verification and ATO preparation
Phase 2: Platform Deployment
Private AI environment provisioning
Security controls implementation
PubMed API integration development
HHS data source connections
ARPA-H document repository integration
User authentication and authorization setup
Phase 4: Training & Rollout
User training for researchers and program managers
Best practices documentation
Use case examples and templates
Responsible AI usage guidelines
Ongoing support procedures
Results & Outcomes
-
60% reduction in literature review time for research teams
-
45% increase in researcher productivity for grant proposal development
-
100% data security compliance with zero security incidents
-
85% user satisfaction rating among ARPA-H researchers
-
Accelerated research hypothesis generation and validation
-
Enhanced cross-disciplinary research connections
-
Improved data-driven innovation capabilities
-
Strengthened health ecosystem connectivity
-
Facilitated successful research transitions from concept to implementation
-
Reduced risk of sensitive information exposure compared to commercial AI platforms
Compliance & Standards
-
Federal Risk and Authorization Management Program compliance
-
Federal Information Security Management Act compliance
-
Security and privacy controls implementation
-
Full authorization maintained
-
Compliance with federal data handling requirements
-
Ethical AI usage guidelines and monitoring
Use Case Examples
-
Researchers used GRACE to synthesize hundreds of research papers on emerging biomedical topics, reducing weeks of manual review to hours of AI-assisted analysis.
-
Program managers leveraged GRACE to draft sections of research proposals, identify relevant prior work, and ensure comprehensive literature citations.
-
Scientists used GRACE to explore cross-disciplinary connections and generate novel research hypotheses based on existing literature and data patterns.
-
Research teams consulted GRACE for methodology recommendations and statistical approach validation for complex biomedical datasets.
-
Staff used GRACE to quickly locate and interpret relevant HHS regulations and federal health policies applicable to research programs.
Key Personnel
Innovation & Impact
-
GRACE represented one of the first secure, mission-specific generative AI deployments in the federal health research space, establishing a model for responsible AI adoption in government.
-
By providing secure AI capabilities, GRACE directly supported ARPA-H's mission to accelerate biomedical breakthroughs and transform health outcomes through advanced technology.
-
The success of GRACE AI inspired DAWNE IT Solutions to develop the Government Employee AI Mastery Program (GEAMP), a comprehensive training initiative to help federal employees across all agencies leverage AI technologies effectively and securely.
-
The project established best practices for federal AI deployment that can be replicated across other agencies seeking to adopt generative AI while maintaining security and compliance.